Is HBI Physical Strength Affected By Hydrogen vs. Reformed Natural Gas?

INTRODUCTION
It is known that the iron and steel industry is the second largest industrial carbon dioxide (CO2 ) emitter, and it accounts for around 10% of the global emissions. In recent years, the steel industry has been putting a major effort into reducing CO2 emissions. Several approaches and technologies are being considered. One of them is direct reduction of iron ore with hydrogen. Pure hydrogen gas (H2) can be used in the direct reduction process. This paper will discuss the quality of hot briquetted iron (HBI) produced with pure H2 compared to HBI produced with reformed natural gas (NG).
BACKGROUND
Among heavy industries, the iron and steel sector ranks first when it comes to CO2 emissions: around 2.6 gigatonnes of carbon dioxide (2.6 Gt CO2 ) annually.1 To meet global energy and climate goals, steel industry emission must decrease by at least 50% by 2050. Achieving this target will require new technologies. Hydrogen-based direct reduced iron (DRI) is one of them. However, that leads to the question of whether the HBI produced from zero carbon DRI, resulting from using pure H2 as the reducing gas, will reach the same targeted parameters, such as product density and strength when compared to HBI produced from DRI produced with reformed NG.
Tests were conducted at the Midrex Research & Development Technology Center (RDTC) to compare physical and chemical properties of HBI made with H2 versus reformed NG. A typical DR-grade oxide feedstock was selected for the tests, which was reduced with H2 and NG in the RDTC’s large reduction furnace (LRF). Both batches of DRI were then briquetted in the RDTC’s commercial-scale briquetting machine.
DISCUSSION
Chemical composition of the oxide feedstock used in the tests is summarized in Table 1. Typical DR-grade oxide pellets with total iron above 67% were used.
Approximately 400 kg of oxide pellets were used to produce each batch of DRI. Either H2 +CO syngas simulating NG base reduction or H2 /N2 mixed gas simulating H2 reduction was used to produce each batch of DRI. The LRF temperature was controlled at ≈800-850°C depending on the clustering tendency of the oxide pellet. The reduced material was cooled down under N2 atmosphere, and the DRI was unloaded from the LRF.
It was observed that the bed temperature drop for H2 reduction was larger than for NGbased reduction, but the H2 case took less time to complete the reduction process than the NG case.
The resulting DRI from each trial (around 300 kg) was reheated above 750°C and subsequently briquetted in a commercial-scale briquetting machine (see Figure 1). The briquettes were approximately the size of commercially available HBI (116 cc, volume), and each briquetting run was approximately two (2) minutes in duration.
TABLE I.
Oxide Feedstock Analysis

FIGURE 1.
Commercial-scale briquetting machine at Midrex RDTC
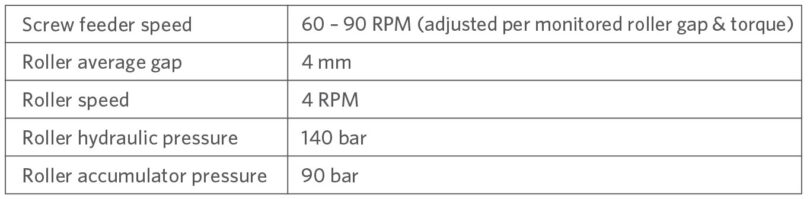
The briquetting machine was set up as follows for both runs:
An example of briquettes produced during the tests is shown in Figure 2.
Chemical analysis of the DRI and HBI was conducted on samples submitted to the analytical laboratory.
DRI and HBI chemical analysis for NG- and H2 -reduced materials is summarized in Table 2. It should be noted that reduction time was different for the NG and H2 runs.
The HBI made from DRI reduced by NG and H2 was evaluated for density, tumble strength, and drop strength. Apparent density was measured for both types of HBI (see Figure 3) in accordance with ISO 15968:2016 by submerging briquettes in water and measuring the weight of HBI before and after soaking. The calculations were applied to determine the density and water absorbency of HBI.
HBI produced with NG had density of 5.1 g/cc, while HBI produced with H2 had density of 5.4 g/cc. Both materials achieved IMO required density of greater than 5 g/cc. The water porosity was measured to be 22.0% for NG-HBI material and 18.9 % for H2 -HBI.
Therefore, it appears that HBI made with H2 has higher density and lower porosity than HBI produced with NG.


FIGURE 2.
Example of HBI produced during the tests
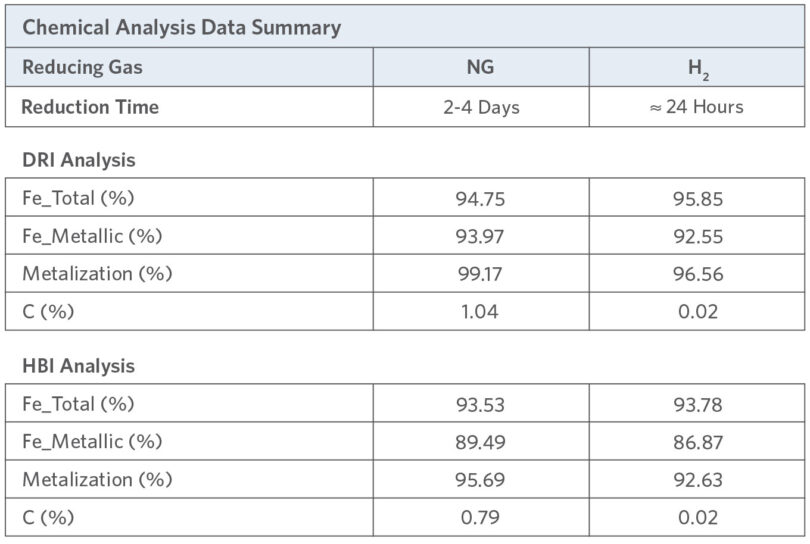
TABLE 2.
Chemical Analysis Data Study
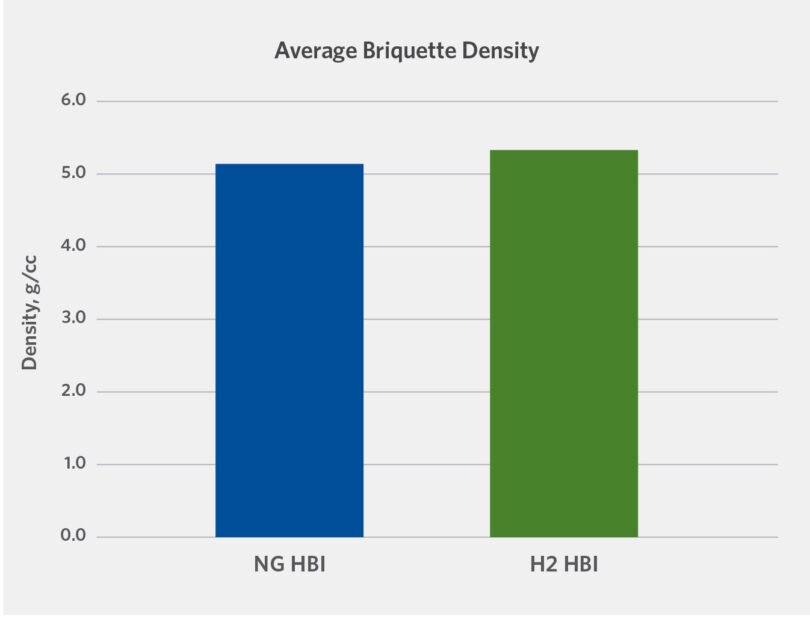
FIGURE 3.
Average briquette density NG-HBI vs. H2 -HBI
The tumble and abrasion indices for both types of HBI were measured in accordance with ISO 15967:2007 in an ISO-style lifter tumble drum with a counter set for stopping at 200 revolutions (N=25 rpm). Sample size was 15+/-0.5 kg of briquettes, and the screen structure was measured and reported.
The drop strength of both types of HBI was evaluated after four (4) drops. The drop height was five (5) m, and the screen structure of the HBI after the drops was reported.
The physical strength for HBI produced with H2 was found to be equivalent to or slightly higher than HBI produced with NG. The results for both tests are summarized in Table 3.
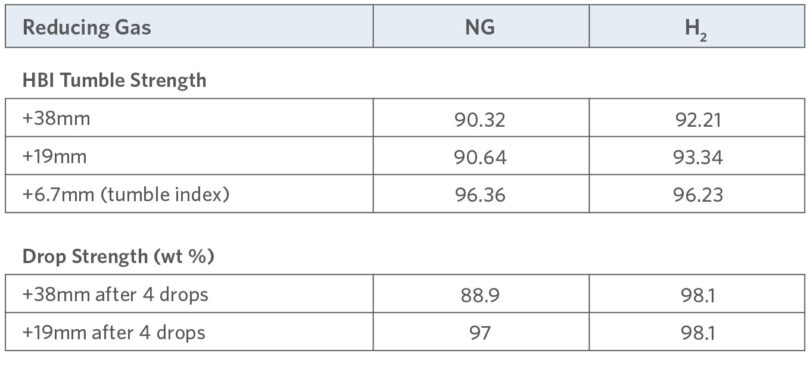
TABLE 3.
Data summary for tumble and drop strength of NG-HBI and H2 -HBI
CONCLUSION
To meet global energy and climate goals, steel industry CO2 emissions must be decreased by at least 50% by 2050. Reducing CO2 emissions from ironmaking process can significantly contribute towards achieving that goal. Shifting from blast furnace-produced iron to reformed NG-produced DRI/HBI can significantly reduce CO2 emissions, and using DRI/HBI produced with pure H2 can reduce emissions even further.
These tests demonstrated that the physical properties of HBI produced from DRI reduced by H2 are very similar to HBI made from NG-reduced DRI. Both HBI types achieved IMO required density of >5g/cc. When comparing HBI made from H2-reduced DRI to the HBI made from NG-reduced DRI, it was observed that the density of H2 -reduced material is slightly higher. H2 -reduced HBI demonstrated acceptable physical strength, with tumble and drop test results similar to or slightly higher than NG-HBI. Therefore, H2 ironmaking using direct reduction technology is a viable first step toward carbon-free steelmaking.
References:
- IEA (2020), Iron and Steel Technology Roadmap, IEA, Paris https://www.iea.org/reports/iron-and-steel-technologyroadmap.
- Midrex Technologies Inc., Direct from Midrex (various publication years).
Acknowledgement
This article was developed from the paper titled “Briquette Strength: Effect of Hydrogen versus Reformed Natural Gas,” by Vita A. Dean, which was presented at AISTech 2023, May 8-11, in Detroit, Michigan. The author thanks her teammates at the Midrex RDTC for their hard work and collaboration in conducting the research on which this article is based.

